Clinical study designs, specifically those that combine Phase 1 and Phase 2 trials, are becoming increasingly common as sponsors pursue more aggressive clinical development timelines. According to Citeline (account required), there are currently 12,469 ongoing (“open”) oncology trials globally, of which 1,660 are combination Phase 1/2 studies (13.31%). This percentage continues to grow year over year.
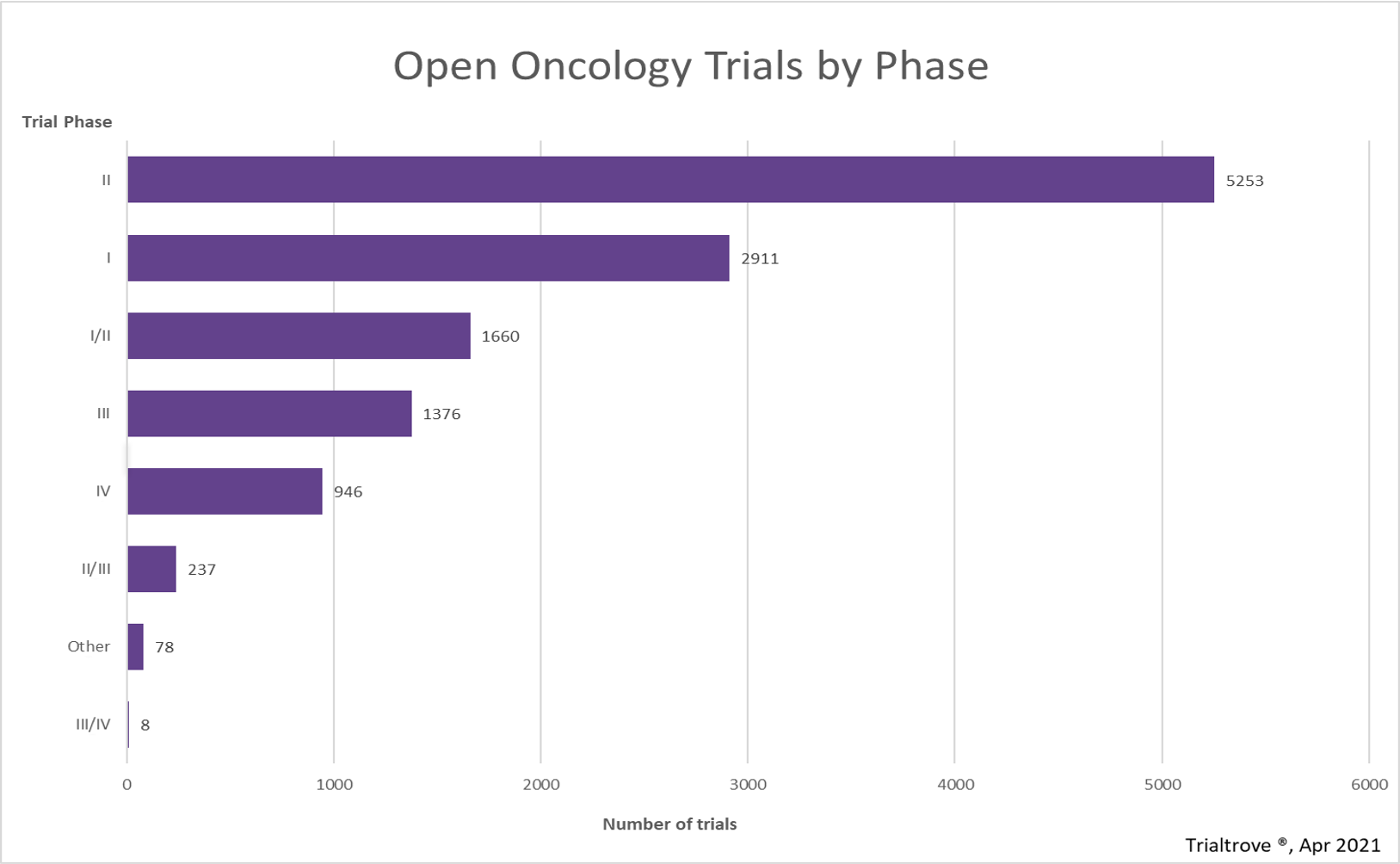
Source: Citeline’s Trialtrove, April 2021.
Managing a dose escalation study is difficult enough, and when you pair it with a Phase 2 study that often requires expansion into additional countries, the complexity of execution goes to another level. In this article, we will review some of our recommended best practices and elements to be aware of when managing a Phase 1/2 combination oncology study and how to effectively and efficiently move from escalation to expansion stages.
Achieving Optimal Recommended Phase 2 Dose
Achieving the optimal recommended Phase 2 dose (RP2D) is critical. This is typically identified following successful completion of your Phase 1 dose escalation stage, which provides the foundation to run the Phase 2 stage that immediately follows. In a Phase 1/2 combination study, this requires not only flexibility in your Phase 1 clinical trial design but, also, you must pay very close attention to the pharmacokinetic (PK) data as well as biopsies to successfully move to the Phase 2 clinical trial stage with the sites you have already waiting for activation. Let’s discuss this further below.
-
Integrating flexibility into your lead-in 3+3 design
While there has been an increase in sponsors adopting more adaptive trial design in early phases, we continue to see several Phase 1 or Phase 1/2 oncology study starting off with the traditional 3+3 design, which requires enrollment of 3 patients within a cohort/dose group. The safety and progress of patients enrolled at each dose level are monitored to ensure no dose-limiting toxicities (DLT) occur before moving onto the next dose and cohort. The process is repeated as we progress through each cohort/dose level.
What often happens however, as the study progresses through each dose level, is usually much more complicated. In reality, there is not enough flexibility built into the Phase 1 design of the combination Phase 1/2 clinical trial protocol. As an example, you can add language to the protocol that states the study will follow a 3+3 design and additional patients may be enrolled (eg, up to 9 patients) at a single dose level. This provides some much needed “wiggle room” so if there is uncertainty about an event being a DLT or you start seeing some interesting response data, you have more room with which to work without having to do a protocol amendment thus slowing recruitment for your study. This approach provides increased flexibility, so you move forward with more robust data and a better understanding of your safety profile.
Here is where that flexibility becomes truly important. -
Ensuring compliance with dose escalation patient PK sampling and biopsies to ensure sufficient data are captured to make key decisions for moving forward
Properly assessing toxicities and finding the optimal RP2D is critical to increasing your program’s probability of success. It is well known and documented that many studies and sponsors have had difficulty identifying the RP2D, which came back to haunt them at later stages in their development.
Collecting sufficient PK and biopsies samples is essential. Take time to review PK data using Precision’s Smart Patient Profiles. Clean your data as you go to ensure accuracy of entered data – this is especially vital for your early subjects. In an environment where every patient, every dose, and every day matter to sponsors, it is critical to take a few extra weeks sometimes to ensure you have PK samples and biopsies collected so these are available for analysis. This may end up saving your organization years in clinical development time and ultimately millions of dollars in the end. Flexible protocol language on cohort enrollment and allowing time for sufficient PK and biopsy sampling will support your efforts in effectively moving to the next stage with the data you need.
Setting up Escalation and Expansion Sites for Success
Managing the mix of sites selected for escalation and which ones will be held in reserve for participation in the expansion stage has its own unique set of challenges that need to be elegantly managed by your team and selected Clinical Research Organization (CRO). Conducting a comprehensive feasibility is a given but is recommended to be done at the beginning of the trial (all sites) instead of sequentially (identifying Phase 1 and later Phase 2 sites). Maintaining your expansion sites’ interest while they are waiting is also critically important, and strategies should be considered during your initial start-up planning. Ramping up expansion sites during escalation and when to do so must also be carefully considered. Also, it is likely that there may be a protocol amendment when moving out from the Phase 1 lead-in stage of your combination study, and it is important to keep that efficient as well. Below, we will discuss all these items in greater detail.
Conduct Full Feasibility at Trial Beginning
Some sponsors and CROs still want to select sites sequentially (stage I and later stage II), but the best practice has shown that full feasibility to examine, tier, and select your sites for escalation vs expansion right at the beginning of your trial is the best method.
- Using the results of your full feasibility, carry out the following:
- Use the results to tier your sites for inclusion in the escalation phase vs expansion. You are likely to use criteria such as:
- Phase 1 dose escalation experience
- Site capabilities (in-house vs external/vendor)
- Site start-up timelines
- Experience in indication
- Ability to manage complex PK and biomarker sampling
- Make sure to assess the competitive environment for tumor types likely to be selected for expansion to understand if an adequate number of sites/regions have been selected or if additional sites/regions need to be onboarded sooner. This assessment should include not only other clinical trials, but also the status of products in late-stage development that are poised for approval during the timeframe of the study.
- Make sure to also identify a few back-up sites for Phase 1 and Phase 2 in case you run into any issues at sites/enrollment snags.
- Use the results to tier your sites for inclusion in the escalation phase vs expansion. You are likely to use criteria such as:
Onboard Expansion Sites During Escalation
Timing on this is critical. Start things too early, and you could have sites waiting for weeks or months. Start too late, and you might lose critical time and valuable recruitment months for your clinical program which translates to potential delays to days on market if/when approved (= revenue). Here are a few things to keep in mind:
- Every study is different. Depending on your study design, make sure your project timeline has modeled out the ideal starting point for your expansion sites by incorporating the start-up timelines for the sites/countries coming onboard for expansion. Make sure your feasibility has appropriate detail/questions about site start-up and contracting.
- It is impossible to know if escalation will go perfectly, but it’s important to make a best estimate on when expansion sites should begin the process for start-up. Make sure to communicate this with your sites so they are not left guessing on status.
- As usual, make sure to get their patient enrollment targets to ensure you can model the full enrollment window for each expansion cohort properly and with a high level of confidence.
Communicate and Engage Expansion Phase Investigators – All Phases
Maintaining interest and motivation with expansion sites can be difficult, especially if you or your CRO are not keeping them apprised of progress with some form of agreed cadence and medium (eg, newsletter, email). During study start-up, make sure to define communication/newsletter expectations with your CRO, as this is truly important for expansion sites in a combination study. A few best practices to consider with regard to site communication:
- Set up a newsletter template or email template for communicating with your escalation and expansion sites. During escalation, keep your expansion sites aware of the progress and news, to maintain their motivation and interest in your study. Remember, they could potentially pick up a competitive study while escalation for your study is ongoing. Communicate, and keep them interested in your study and progress.
- If expanding in certain tumor types, share early response data from dose escalation in those tumor types to generate interest from Principal Investigators (PIs) to facilitate faster expansion enrollment.
- Get escalation PIs on a call with newly added expansion PIs to facilitate knowledge transfer.
- An example would be to bring in some of your new PIs to your existing safety calls with your escalation PIs so dialogue can occur, and they can hear and catch up. If this is not possible, then setting up a dedicated/focused conversation on data sharing is helpful to increase PI interest.
- Communicate the number of slots remaining in each dose expansion cohort as this shares progress and availability and can also spark healthy competition between sites.
- Also share new responses in these emails to further gain excitement/traction for increased enrollment.
- Share any planned publications or conference abstracts/presentation data for on-trial progress reports in order to continually attract new patients.
Managing Protocol Amendments
Protocol amendments are still likely to happen, even after you build in flexibility to your 3+3 per best practice covered earlier in this article. Per Tuft’s article from 2017, your study is highly likely to need at least one, if not more, protocol amendments. Below are a few recommendations to maintain momentum with your expansion sites while completing a protocol amendment between Phase 1 stage completion and Phase 2 first patient-in:
- You can open sites under the initial protocol and do a quick Institutional Review Board (IRB) – ethics committee revision to minimize delays in approval.
- Time this submission to align with continuing review or updated Investigator’s Brochure (if planned) to minimize number of IRB-ethics submissions needed.
- Track all protocol-related concerns or difficult inclusion/exclusion during dose escalation so they can be amended before expansion, if there is a planned protocol amendment.
Conclusion
Combination Phase 1/2 studies are complex but entirely executable with appropriate planning, coordination, and communication. The number and percentage of these types of studies, per Citeline, is growing as more sponsors turn to combination studies to expedite their development timelines. It is critical to work with a CRO, like Precision for Medicine, that runs these studies en masse, and understands the intricacies of execution as discussed in this article. Precision for Medicine is a global CRO that excels at managing complexity while specializing in personalized medicine, drug development, and early phase oncology. Speak with our team today; we would be delighted to support you in your next combination Phase 1/2 clinical trial.