Cell therapies are advanced treatments that must be administered at accredited centers with experience and established quality processes, procedures, and practices to ensure patient safety. As of January 2021, 298 centers have been accredited by the Foundation for the Accreditation of Cellular Therapy (FACT) in the United States and 274 centers have been accredited by the Joint Accreditation Committee ISCT-Europe & EBMT (JACIE) in the European Union, though not every center has administered cell therapy.1,2
Research activity in the cell therapy field is brisk, with approximately 1900 studies either ongoing or planned, but the majority of clinical trial conduct is concentrated at established high-profile sites. Developers seeking to accelerate their research may benefit from looking beyond the go-to institutions to lesser-known sites that have the bandwidth, access to patients, infrastructure, and systems to conduct cell therapy studies. Contract research organizations (CROs) with cell therapy experience play a pivotal role in qualifying and supporting these under-the-radar sites to help ensure clinical trial success.
Uncovering Hidden Gems
Data from Citeline Sitetrove show that, to date, over 5000 cell therapy studies have been conducted globally. Over 4100 sites have been involved in industry-sponsored cell therapy research, but the majority of studies tend to be concentrated among a handful of high-profile sites.3 Looking across regions in both the United States and Europe, there are a multitude of “hidden gems” — centers with the necessary infrastructure and qualifications to conduct cell therapy trials that are being overlooked by developers.
Figure 1. Distribution of US sites involved in cell therapy trials
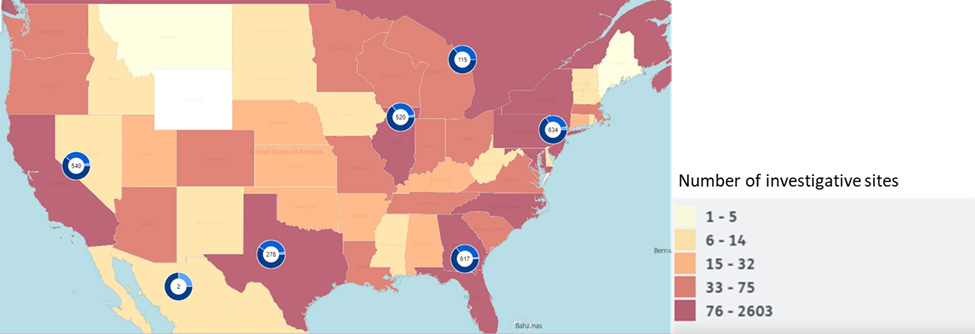
Adapted for Citeline Sitetrove
These under-the-radar sites are leading academic medical centers and qualified community-based hospital systems with the capability and capacity to conduct resource-intensive cell therapy studies. With fewer competing trials ongoing at these centers, developers may find it easier to locate champions for their studies, create clinical interest, and move forward quickly.
Selecting eligible, but lesser-known sites also addresses another barrier associated with cell therapies —access. Many of these FACT- or JACIE-accredited centers are located in areas such as the US Midwest, that are currently underserved by cell therapy, giving patients in these areas the opportunity to participate in clinical trials. Notably, there are approximately 200 transplant centers in the United States, but fewer than 100 of them are certified to administer cell therapies, underscoring the potential to expand availability of these advanced therapies to a much broader population.4
Helping Overlooked Sites Become Cell Therapy Centers of Choice
Partnering with a CRO like Precision for Medicine that is aware of — and has experience working with —these hidden gems helps developers avoid the waitlists at the go-to centers and focus on getting sites up and running. Given that developers may be less familiar with these centers, a higher level of scrutiny may be applied to qualifying them as investigative sites. CROs play a pivotal role in supporting these sites to meet that burden of proof.
The patient and product journeys involved in the delivery of cell therapies are complex and require close coordination of teams within and external to the site. As an example, for an autologous-based therapy, after being enrolled in a clinical trial, patients proceed through raw material collection. That raw material is transported to a contract development and manufacturing organization (CDMO) for engineering before being returned to the site for administration. The patient may undergo lymphodepletion prior to receiving the cell therapy, and will require close monitoring for potential toxicities after administration. Following this, the patient will continue to be monitored long-term, in some instances up to 15 years.5 As timing is critical, managing the overall process in order to assure proper cell therapy delivery to that patient requires tight coordination among the sites, laboratories, cell labs, CDMOs, and developers. Establishing a strict chain of custody in order to maintain chain of identity and instituting multi-stakeholder communication touchpoints are essential components of site support.
Precision for Medicine has established rigorous processes for supporting sites and clinical trial success, including:
- Reviewing existing institutional policies and establishing a baseline on site knowledge and experience
- Developing a site onboarding curriculum, including product manuals and process checklists for every step of the patient and product journeys, helps ensure that all procedures are executed on time and according to plan for every patient
- Providing a trial initiation kit that includes trial-specific training modules for study personnel as well as other staff members who may be involved with the study (eg, apheresis center, surgical team, and intensive care unit staff); product lifecycle flow charts, including communication flow with project team; adverse event management pocket cards; product information; and any product-specific resources
- Devising a detailed, study-specific apheresis manual to ensure protocol compliance. Many of these lesser-known sites have previous experience with hematopoietic cell transplantation and are likely to have established standards for procedures such as apheresis. However, every cell therapy trial is unique, so it is important to understand how site level clinical workflows may need to be adapted or adjusted to meet study requirements
- Ensuring toxicity management protocols are in place and site staff are appropriately trained. Cell therapies have the potential for causing life-threatening toxicities, so the ability to implement cytokine release syndrome and neurotoxicity management protocols is critical
- Performing dry runs during the site initiation visit, from procurement of the raw material to receipt of the product at the site for administration to the patient. These practice runs are useful not only for identifying and mitigating potential risks (eg, chain of custody established, paperwork completed correctly, etc), but also for increasing the competence and confidence of site staff
- Putting in place processes for ongoing site training and certification keeps staff engaged and helps reduce site burden
Engaging with and supporting sites in this manner not only helps centers get up to speed on what being a site would entail, but also allows sponsors and CROs to assess and confirm the center’s readiness to be a high-performing site.
Key Takeaway
Providing comprehensive support to sites is a critical factor for cell therapy clinical trial success. Developers who are open to uncovering and supporting hidden gems will find investigators and site staff who are enthusiastic about their research and poised to become centers of choice for the administration of advanced therapies. Partnering with a CRO that is well-versed in the challenges and opportunities of these complex, resource-intensive studies can help developers polish these sites to their full potential.
This article was originally published in Cell & Gene
1 Foundation for the Accreditation of Cellular Therapy (FACT). Search for a FACT Accredited Organization. Available at https://www.ebmt.org/jacie-accredited-centres. Accessed January 30, 2021.
2 European Society for Blood and Marrow Transplantation (EBMT). JACIE Accredited Centres. Available at https://www.ebmt.org/jacie-accredited-centres. Accessed January 30, 2021.
3 Citeline SiteTrove. Search Parameters “CAR-T, TCR, TIL, or Cell Therapy, Other”; Sponsor Type “Industry.” January 28, 2021.
4 Kansagra A, Farnia S, Majhail N. Expanding access to chimeric antigen receptor T-cell therapies: challenges and opportunities. Am Soc Clin Oncol Educ Book. 2020;40:1-8.
5 Elverum K, Whitman M. Delivering cellular and gene therapies to patients: solutions for realizing the potential of the next generation of medicine. Gene Ther. 2020;27:537-544.